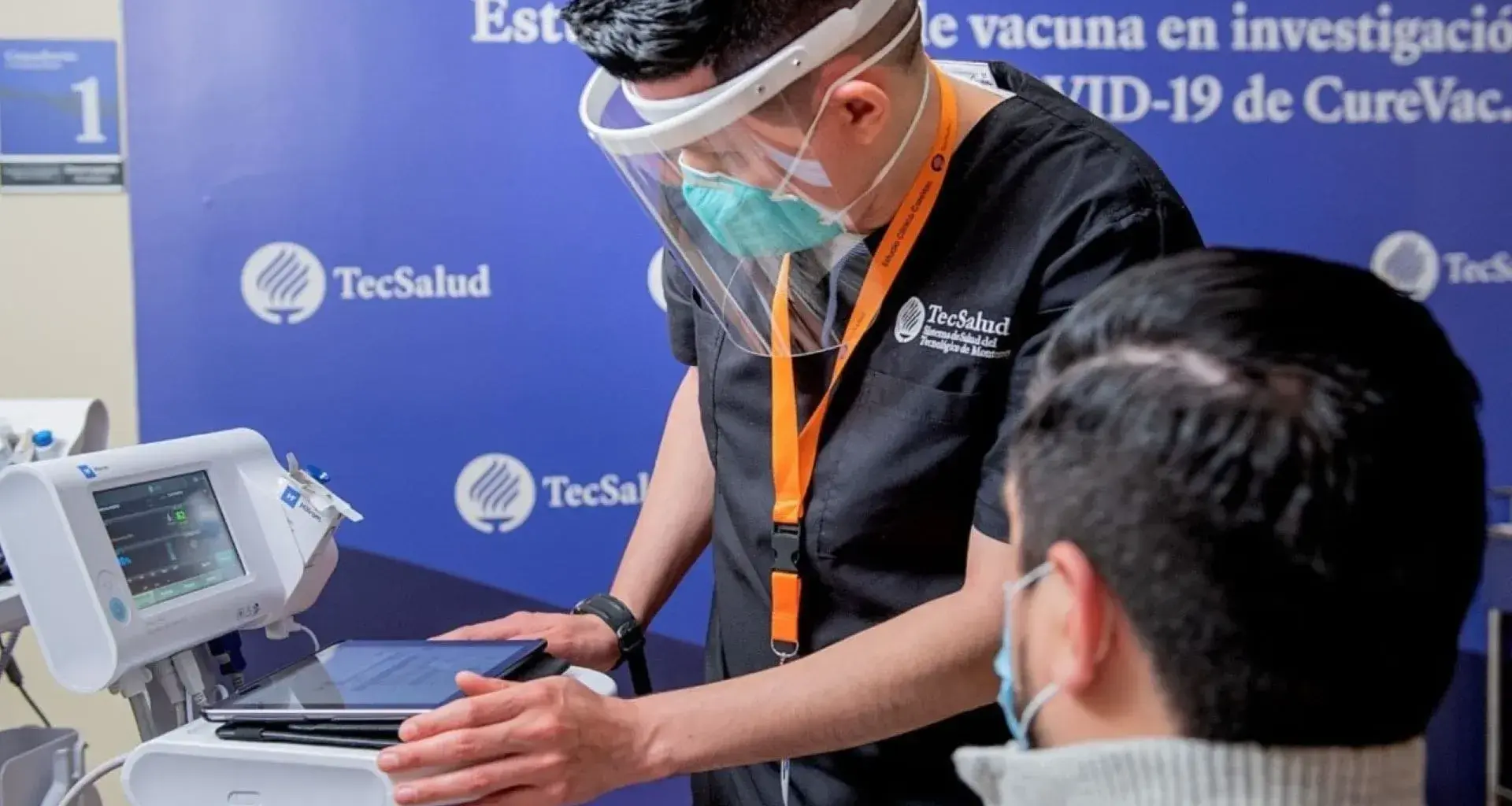
“We have shown that things can be done well and on a large scale in Mexico,” said Michel Martínez, head of the TecSalud Epidemiological Surveillance Unit and leader of TecSalud’s COVID-19 Program.
TecSalud, the health system of Tecnológico de Monterrey, demonstrates research leadership by participating in the German CureVac COVID-19 vaccine clinical trial.
“The vaccine uses the messenger RNA principle. There are three vaccines of this type worldwide and two, Pfizer and Moderna, have already been authorized. CureVac is in the process of being authorized and is going through phase II and phase III trials,” explained Michel Martínez, who is Principal Investigator for the trials.
“TecSalud is participating in a phase III trial, which will evaluate the efficacy of this vaccine in preventing SARS-CoV-2 infections and reducing COVID-19 symptoms, hospitalization, and deaths of people who become infected,” he added.
Additionally, Martínez stressed the importance of Mexico participating in such large phase III trials. To do so, it has implemented infrastructure and trained health workers.
“The clinical data we have gotten from volunteers will help COFEPRIS and international organizations determine if it is possible to authorize the new CureVac COVID-19 vaccine for emergency use. This improves the profile of our country and our institution,” explained Servando Cardona, National Director of Clinical Research at TecSalud.
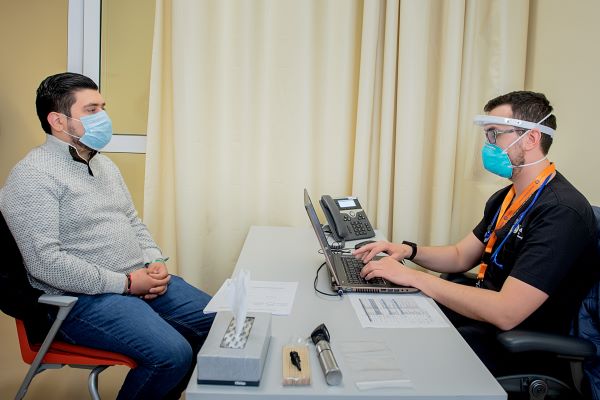
TecSalud leads global recruitment of volunteers
The Zambrano Hellion Hospital, in San Pedro Garza García, N.L., was selected as the site which would carry out the vaccination of volunteers and provide follow-up on the candidate vaccine’s clinical trial.
In total, TecSalud recruited 1,973 volunteers, who have already received either a placebo or the vaccine.
“Randomization was 1:1. We were the number one center globally, with a difference of more than 200 volunteers from the second-place location,” said Martínez.
“The follow-up of almost 2,000 volunteers during the 13 months of the trial is an enormous logistical task. It’s one thing to recruit them, but what makes it complicated is keeping them in the study until the authorities decide to open it up or shut it down. That’s also one of the parameters for evaluation,” said Cardona.

Advanced technology in many medical fields
Messenger RNA technology can be applied in many fields beyond just infectious diseases. “It can also be used in oncology and cardiology, where research is already being done on heart failure problems,” said Michel Martínez.
“In terms of infectious diseases, the use of this technology for viral diseases is not new. It has already been used on Ebola and rabies; it’s already come a long way,” he stressed.
He also explained that such technology shortens vaccine development times, which allows vaccine modifications to be made quickly and safely to keep up with new COVID-19 variants.
“Being able to carry out studies of this size, providing high quality monitoring of volunteers, and complying with all aspects of data registration that the sponsor requires has been a great opportunity so far and also a pleasant experience with favorable results,” explained Cardona.
“When the sponsor determines that the vaccine gives better results than the placebo, those volunteers who took part and received the placebo will be immediately placed in the vaccine group and will receive the vaccine. We thank all those who collaborated in finding a solution to this pandemic,” he concluded.

READ MORE NEWS AT CONECTA: